By Tom Latek
Kentucky Today
The FDA and CDC, in collaboration with state and local partners, continue investigating a multistate outbreak of infant botulism, which has now sickened nearly two dozen children, including one case in Kentucky.
Epidemiologic and laboratory data show that ByHeart Whole Nutrition infant formula might be contaminated with Clostridium botulinum, which is causing infant illness in multiple regions of the country.
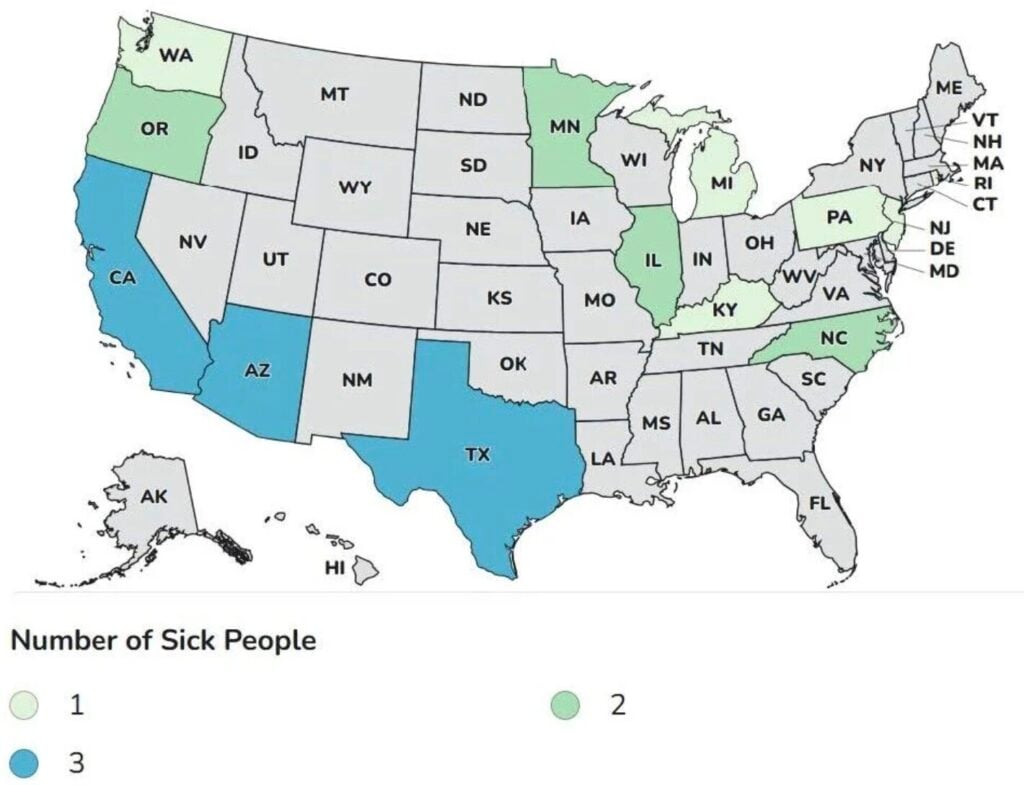
As of November 14, a total of 23 infants with suspected or confirmed infant botulism and confirmed exposure to ByHeart Whole Nutrition infant formula (various lots) have been reported from 13 states (see map). Laboratory confirmation for some cases is ongoing.
For 22 cases with illness onset information available, illnesses started on dates ranging from August 9 to November 11. All 23 infants were hospitalized. No deaths have been reported to date. For 22 infants with age and sex information available, they range in age from 16 to 200 days and 10 of them, or 45 percent, are female.
As part of this investigation, officials in several states have collected leftover infant formula for testing. On November 8, 2025, preliminary laboratory results reported by the California Department of Public Health suggest the presence of the bacteria that produce botulinum toxin in an open can of ByHeart infant formula (lot 206VABP/251131P2) that was fed to an infant with infant botulism.
Besides the case in Kentucky, other states with cases include Arizona (3), California (3), Illinois (2), Michigan, Minnesota (2), North Carolina (2), New Jersey, Oregon (2), Pennsylvania, Rhode Island, Texas (3) and in the state of Washington.
Additional testing is underway, and results are expected in the coming weeks. Detection of Clostridium botulinum in infant formula is difficult, and a negative test result does not rule out the presence of the bacteria in the product.
FDA’s investigation, including onsite inspections and sample collection, is ongoing to determine the point of contamination.
The CDC and FDA recommend that parents and caregivers should not use ByHeart Whole Nutrition Infant Formula containing lot numbers 206VABP/251261P2 and 206VABP/251131P2, both with use by dates of Dec, 1, 2026, and should throw the product away immediately.
If your child is experiencing symptoms after consuming ByHeart Whole Nutrition Infant Formula and you still have the formula in your home, please record the information on the bottom of the package before throwing it away.

















